 |
| modifying recombinant genes | expression in plants | risks and safety | |
 | |
|
Section Navigation
» general methodology:
» modifying recombinant genes:
» expression in plants:
» risks and safety: |
|
← back preparing genes for expressionModifying genes for transgene expressionWhether transgenic plants are required for the production of recombinant cytokines, antibodies, or antigens, the process is usually very similar. There are two major ways of getting a foreign gene of interest into the plant: (1) by Agrobacterium-mediated transformation and (2) by gene gun transfer[1]. In addition to these two major systems, some new viral vector methods, namely the tobacco mosaic virus, are currently being explored for the production of antibodies in plants [2]. However, their efficiency has not been thoroughly established. Therefore, our review will discuss the first two conventional transformation methods only. Before either the Agrobacterium-mediated transformation or the gene gun transfer can be employed, the transgene itself must be isolated and cloned. In our case, this would involve isolating a mammal cytokine or antibody gene (usually human) and ensuring that they are ready for transgene expression. For example, a medically important cytokine that has been previously expressed in transgenic plants is interleukin-12. Natural human IL-12 is made up of two chains; an α-chain and a β-chain. These two chains are encoded by disparate genes. These chains only associate after translation and gain functionality then. However, high β-chain concentrations can lead to unwanted β-homodimers, which repress the natural heterodimer's activity. Therefore, IL-12 subunit concentrations must be tightly controlled. Scientists have circumvented this problem by expressing the recombinant IL-12 molecule as a single-chain fusion polypeptide, in which the α- and β-chains are kept together by a linkage sequence. This means the two different genes are placed next to each other on the transgene cassette with a linkage sequence in between, so that the protein is translated as one molecule instead of two halves[3]. For antibodies, since natural immunoglobulin genes require complicated rearrangements to generate their wide array of specificities and five isotypes (IgM, IgD, IgA, IgE, IgG), often recombinant antibodies may take only a part of the pre-arranged gene locus, say only the parts of the locus relevant to encoding IgM [4], depending on which molecule scientists are interested in. Sometimes antibody derivatives are desired instead of the entire natural immunoglobulin, for example Fab fragments[1]. Transgene Constructs Both transformation methods mentioned above (elaborated on the next page) require a full transgene construct. Namely, the gene of interest needs a promoter to regulate its expression and a polyadenylation signal to stabilise its mRNA. The most popular promoter by far is the CaMV (cauliflower mosaic virus) 35S promoter, which allows increased and constitutive production of the transgene in all tissues of the plant (i.e. it is not tissue-specific). Oft-used polyadenylation signals include the Agrobacterium tumefaciens nos gene (for nopaline synthase) and the ssu gene (for small subunit) from the pea[1]. (See diagram below.) However, depending on the nature of the intended expression, this may change.
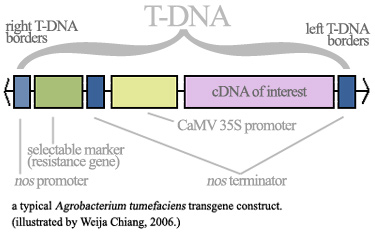 In a study in which IL-2 was expressed in potato tubers, an alternative promoter known as the patatin promoter was used that resulted in not only higher production than the CaMV 35S promoter, but also directed protein expression in a tissue-specific manner to the microtubers of the potato[5]. Finally, it must also be recognised that plants provide natural storage sites in their seeds. Seeds, unlike extracts, or fruits, are hardier and last longer, surviving transport and long periods of storage. Studies have managed to express human granulocyte macrophage colony stimulating factor (hGM-CSF) in the seeds of tobacco plants using a rice endosperm-specific glutelin promoter[6].
→ next references1) Ma JK, Drake PM, Christou P. The production of recombinant pharmaceutical proteins in plants. Nat Rev Genet 2003, 4: 794-805.2) Schillberg S, Twyman RM, Fischer R. Opportunities for recombinant antigen and antibody expression in transgenic plants--technology assessment. Vaccine 2004, 23: 1764-1769. 3) Gutierrez-Ortega A, Avila-Moreno F, Saucedo-Arias LJ, Sanchez-Torres C, Gomez-Lim MA. Expression of a single-chain human interleukin-12 gene in transgenic tobacco plants and functional studies. Biotechnol Bioeng 2004, 85: 734-40. 4) Rusconi S, Köhler G. Transmission and expression of a specific pair of rearranged immunoglobulin and genes in a transgenic mouse line. Nature 1985, 314: 330-334. 5) Park Y, Cheong H. Expression and production of recombinant human interleukin-2 in potato plants. Protein Expr Purif 2002, 25: 160-5. 6) Sardana RK, Alli Z, Dudani A, Tackaberry E, Panahi M, Narayanan M, Ganz P, Altosaar I. Biological activity of human granulocyte-macrophage colony stimulating factor is maintained in a fusion with seed glutelin peptide. Transgenic Res 2002, 11: 521-31.
|